Investigational Drug Development
Looking Forward
Fujifilm is conducting research to study the potential of new drugs to address unmet medical needs, focusing on cancer, infectious diseases and central nervous system diseases.
Fujifilm currently has clinical trials in the United States for cancer. These clinical trials are underway in collaboration with various institutions throughout the country.
*Avigan Tablet (favipiravir) has not been approved by the FDA for use in the USA
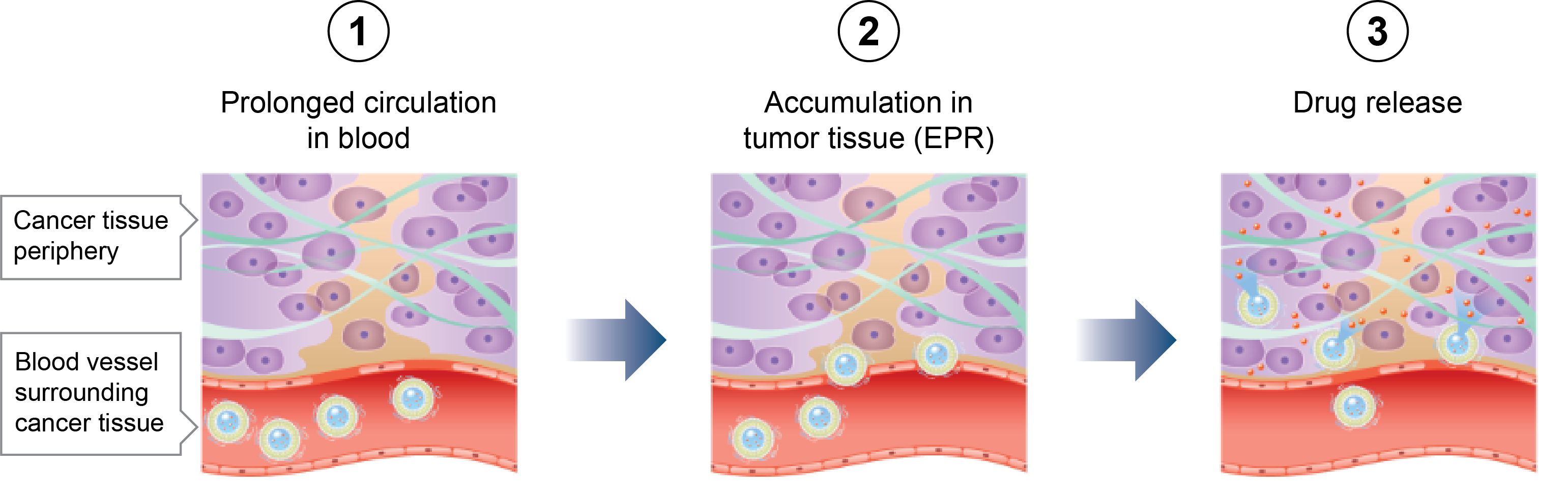
Investigational drug pipeline
Infectious diseases
| Code | Mode of Action | Indication | Region | Stage | Clinical Trial Information |
| T-705 | RNA polymerase inhibitor | Influenza | Japan | Approved | https://clinicaltrials.gov/ct2/results?cond=&term=T-705&cntry=&state=&city=&dist= |
| Severe fever with thrombocytopenia syndrome | Japan | Phase 3 | |||
| T-4288 | Bacterial protein synthesis inhibitor | Otorhinolaryngologic infections | Japan | IND filing | |
| Respiratory tract infections | Japan | Phase 3 |
Oncology
| Code | Mode of Action | Monotherapy or Combination | Indication | Region | Stage | Collaboration | Clinical Trial Information |
| FF-10502-01 | DNA polymerase inhibitor | Advanced solid tumors | U.S.A. | Phase 2 | https://clinicaltrials.gov/ct2/results?cond=&term=FF-10502-01&cntry=&state=&city=&dist= | ||
| FF-10832 | Liposomal gemcitabine (DNA replication polymerase inhibitor) |
Monotherapy | Advanced solid tumors (Biliary tract cancer) |
U.S.A. | Phase 1 | https://clinicaltrials.gov/ct2/results?cond=&term=FF-10832&cntry=&state=&city=&dist= | |
| Combination with pembrolizumab1 | Advanced solid tumors (Non-small cell lung cancer and urothelial cancer) |
U.S.A. | Phase 2 | ||||
| FF-10850 | Liposomal topotecan (topoisomerase I inhibitor) |
Advanced solid tumors (Ovarian cancer and Merkel cell carcinoma) |
U.S.A. | Phase 1 | https://clinicaltrials.gov/ct2/results?cond=&term=FF-10850&cntry=&state=&city=&dist= |
1Fujifilm Begins U.S. Clinical Phase 2a Study of FF-10832 in Combination with KEYTRUDA® (pembrolizumab, Merck & Co., Inc., Rahway, N.J., U.S.A.) for Patients with Advanced Solid Tumors | Fujifilm [United States]
*Merck & Co., Inc. is known as MSD outside the United States and Canada.
Neurology
| Code | Mode of Action | Indication | Region | Stage | Clinical Trial Information |
| T-817MA | Neuroprotectant, microglial modulator, neuroplasticity facilitator | Alzheimer’s disease | Japan U.S.A. EU |
Phase 2 | https://clinicaltrials.gov/ct2/results?cond=&term=T-817MA&cntry=&state=&city=&dist= |
| Functional recovery after stroke (Promoting the effect of rehabilitation) | Japan | Phase 2 |
Expanded access/Compassionate use
Currently, FUJIFILM Pharmaceuticals U.S.A., Inc. is not accepting expanded access/compassionate use requests.
Partnership Strategy
We are seeking the following partners:
- Companies interested in clinical development and commercialization of Fujifilm’s pipeline drugs.