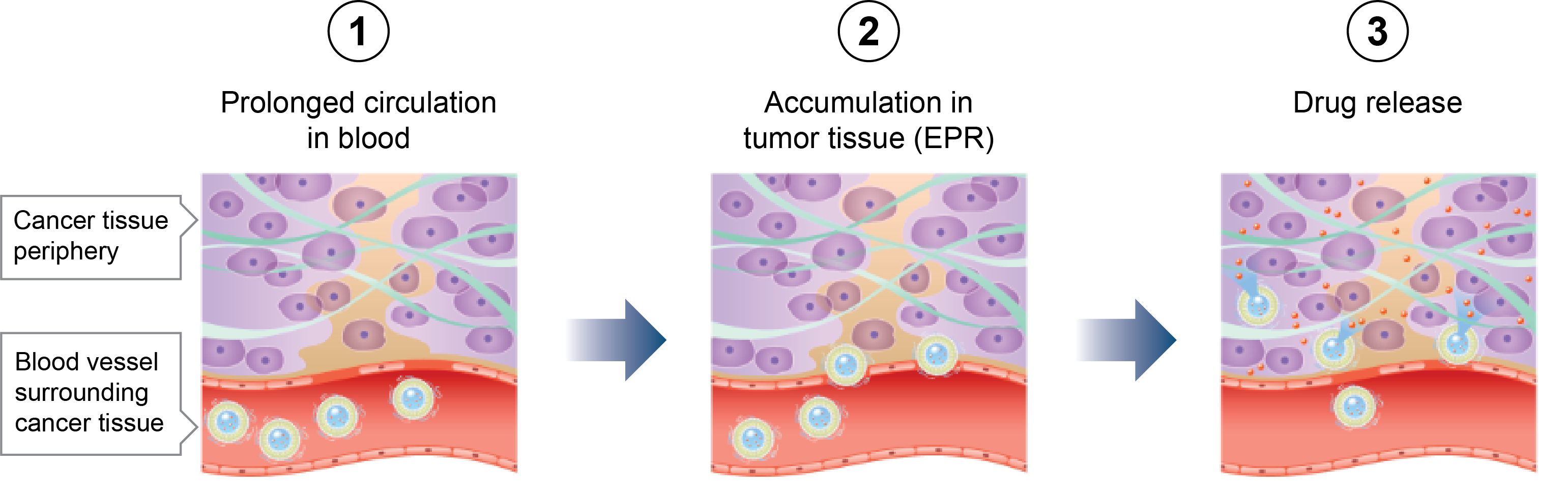
The decision of the European Commission (EC) on the approval is expected in September 2020, which would grant Centus the marketing authorization in 27 European Union (EU) member states, the United Kingdom (UK) and the European Economic Area (EEA) member states of Norway, Iceland and Liechtenstein.
Centus President Hiroshi Ohashi, Ph. D. commented, “We are happy to receive a positive CHMP opinion toward the approval of EquidacentTM, our bevacizumab biosimilar. We will continue to make every effort to obtain the approval for EquidacentTM, which could help patients and healthcare professionals.”
Fujifilm Kyowa Kirin Biologics President and CEO Atsushi Matsumoto, Ph. D. said, “I am delighted that CHMP decided to recommend the approval of the proposed biosimilar bevacizumab. We will continue our efforts to bring high quality and affordable biosimilars to patients throughout European countries.”
Centus was established in 2015 as a joint venture between Fujifilm Kyowa Kirin Biologics and AstraZeneca. Fujifilm Kyowa Kirin Biologics has granted an exclusive license to Centus for the development, manufacture and commercialization of EquidacentTM on a worldwide basis. Centus has been promoting clinical development of EquidacentTM.
Data submitted as part of the Marketing Authorization Application for EquidacentTM included similarity assessment in analytical testing, preclinical and clinical studies that demonstrated biosimilarity to the bevacizumab reference product, Avastin®. The phase 3 clinical study, AVANA, conducted by Centus, demonstrated no clinically meaningful differences in terms of safety, efficacy and immunogenicity compared with the reference product, Avastin®, in non-small cell lung cancer patients.
About Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody that blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A). It reduces growth and metastasis of several solid tumors.
About Centus
Centus Biotherapeutics (Centus) founded in 2015 is a joint venture between AstraZeneca, a global pharmaceutical leader, and Fujifilm Kyowa Kirin Biologics, a Japanese biopharmaceutical company focused on biosimilar development.
With the unique coupling of world class biologics expertise, global commercial infrastructure and deep biosimilar development experience, Centus is well positioned to win the trust of doctors and serve the needs of patients worldwide.
For more information please visit: https://www.centusbiotherapeutics.com
About Fujifilm Kyowa Kirin Biologics
Fujifilm Kyowa Kirin Biologics was established by FUJIFILM Corporation (President: Kenji Sukeno; “Fujifilm”) and Kyowa Kirin Co., Ltd. (President and CEO: Masashi Miyamoto, “Kyowa Kirin”) on March 27, 2012 as a company for developing, manufacturing, and marketing biosimilars. Its pipeline includes EquidacentTM and Hulio®, an adalimumab biosimilar (Product Code: FKB327), a drug used to treat a range of inflammatory diseases.
By merging the technologies in advanced production, quality control and analysis which Fujifilm has developed over many years through its photographic film business, with the proprietary technologies and know-how which Kyowa Kirin has accumulated through its biopharmaceutical R&D and manufacturing, Fujifilm Kyowa Kirin Biologics creates revolutionary production processes and reduces costs for the production of biosimilars. Through this partnership, the company will develop and manufacture reliable, high quality, cost-competitive biosimilar products and commercialize these products in a timely manner. With this strategy, Fujifilm Kyowa Kirin Biologics aims to hold a leading position in the expanding biosimilar market.
You can learn more about the business at: fujifilmkyowakirin-biologics.com.
For inquiries on information in this media release, contact:
Fujifilm Kyowa Kirin Biologics
Ryohei Kawai
Kyowa Kirin Co., Ltd (for Fujifilm Kyowa Kirin Biologics)
+81-3-5205-7205
Email: media@kyowa-kirin.co.jp
Takuro Nishijima
FUJIFILM Holdings Corporation (for Fujifilm Kyowa Kirin Biologics)
+81-3-6271-2000
###