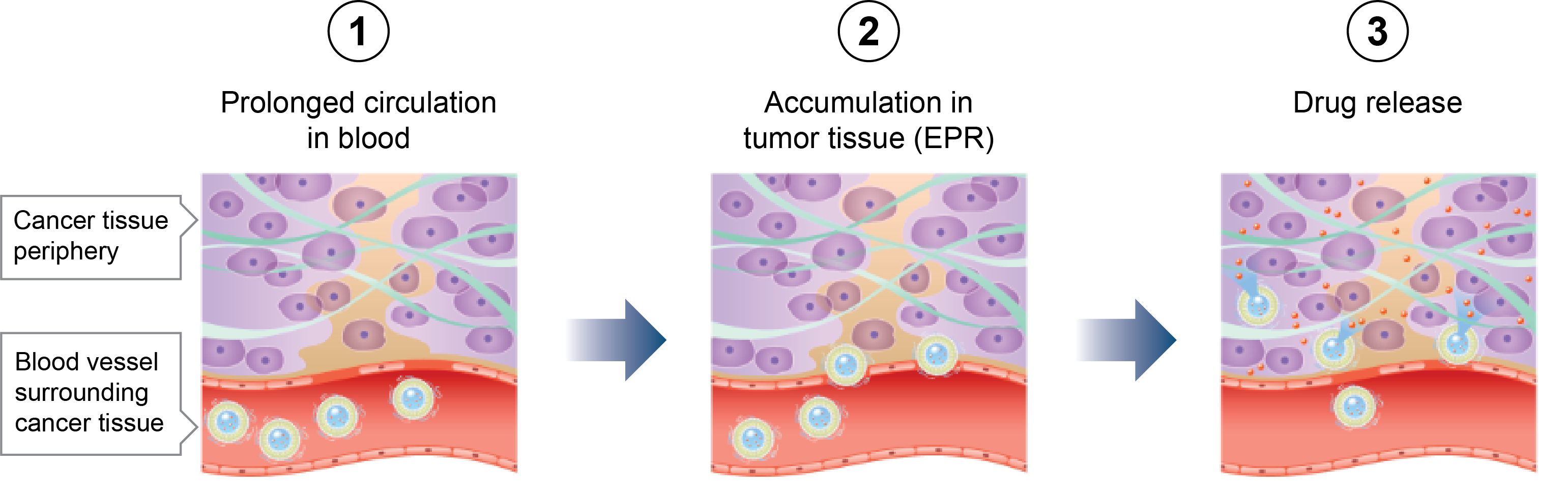
F-1515 is an agent for peptide receptor radionuclide therapy (PRRT), a type of radioligand therapy (RLT)*1, using a somatostatin*2 analog labeled with a radioisotope, lutetium-177 (177Lu). Neuroendocrine tumors originate in neuroendocrine cells that secrete hormones and peptides. They develop in a variety of organs throughout the body, in particular the pancreas, gastrointestinal tract, and lungs. Many neuroendocrine tumors are diagnosed in relatively advanced stages. Once the disease has advanced to stages where surgical resection—the first-line treatment—is no longer possible, drug therapy is selected but choice is limited. It is therefore a disease with a high unmet medical need.
In 2015, FUJIFILM Toyama Chemical concluded a licensing agreement with Advanced Accelerator Applications International S.A. (hereinafter “AAA”), a Novartis company, for the domestic development and marketing of F-1515, also known as Lutathera® in markets where the drug is already approved. Lutathera® is currently approved in various countries and regions including 32 European countries, the United States, Canada, Israel, South Korea, Singapore and Hong Kong. Since then, FUJIFILM Toyama Chemical has carried out clinical development of the drug as “F-1515.”
A phase I clinical study and a phase I-II clinical study conducted recently in Japan confirmed the drug’s efficacy and safety in Japanese patients with somatostatin receptor positive, pancreatic, gastrointestinal, and pulmonary neuroendocrine tumors.
Together with F-1515, the company also filed an application for marketing authorization of LysaKare®*3 (INN: L-lysine hydrochloride/L-arginine hydrochloride. Domestic development code: F-1520), an amino acid solution used during administration of Lutathera®, whose domestic development and marketing rights have been obtained from AAA.
By adding a radiopharmaceutical therapeutic F-1515 to the OctreoScan® Injection Kit (for the preparation of indium pentetreotide [111In] injection), a radiopharmaceutical diagnostic for neuroendocrine tumors that is already being marketed in Japan, FUJIFILM Toyama Chemical will expand its offer of comprehensive solutions for disease management, from diagnosis imaging to therapy.
Going forward, FUJIFILM Toyama Chemical will continue to contribute to enhancing medicine even further by delivering high value-added drugs.
*1 A type of therapy in which ligands, or targeting molecules that specifically bind to receptors expressed by a tumor are labeled with radioactive substances and administered to patients to irradiate the target foci from inside the body. PRRT is a style of RLT.
*2 A peptide hormone consisting of fourteen amino acids that are produced in the hypothalamus, the pituitary gland, as well as the delta cells in the pancreatic islets of Langerhans. It has actions that inhibit the secretion of growth hormone, insulin, etc. Because somatostatin receptors are highly expressed in neuroendocrine tumors, somatostatin is considered one of the effective targets of neuroendocrine tumor treatment drugs.
*3 An amino acid solution for reduction of renal (kidney) radiation exposure during therapy with Lutathera®.
[About FUJIFILM Toyama Chemical Co., Ltd.]
FUJIFILM Toyama Chemical Co., Ltd. conducts the research, development, manufacture, and sales of radiopharmaceuticals and small molecule pharmaceutical products. Under close cooperation with FUJIFILM Corporation, it aims to develop innovative diagnostic and therapeutic radiopharmaceuticals, as well as therapeutic drugs having unique mechanisms of action in the fields of “oncology,” “central nervous system diseases,” and “infectious diseases” where significant unmet medical needs still exist. It also works to develop new medicines utilizing drug delivery system (DDS) technologies designed to deliver the required amount of a drug in a timely manner to a specific area of the body. By exploring synergy with in vitro diagnostic devices and reagents owned by Fujifilm group companies, the company will expand its offering of comprehensive solutions from diagnosis to treatment. FUJIFILM Toyama Chemical has expanded its business areas to medical IoT solutions including a system to support pharmacists’ drug dispensing auditing work, and a transportation device that enables strict temperature control suited to blood products, cells, and tissue used for regenerative medicine. Through the development and supply of high-quality, high added-value new drugs and products that support clinical settings, FUJIFILM Toyama Chemical strives to solve various social challenges and contribute to improving medicine and enhancing quality of life. For more information, please visit http://fftc.fujifilm.co.jp/en/
|
For inquiries on information in this media release, please contact: |
###